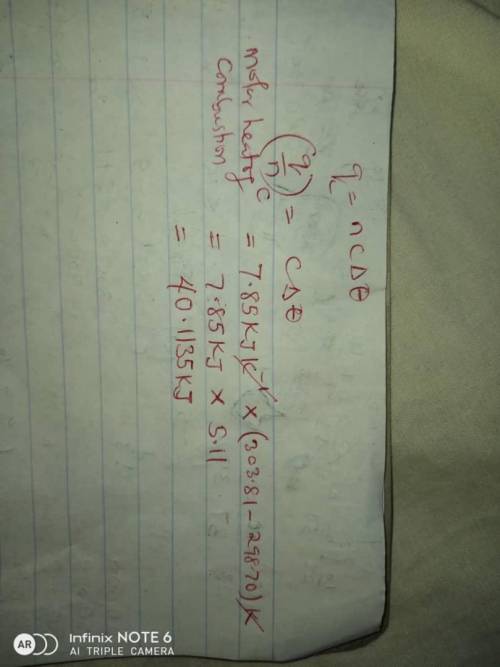Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
A1.11 g sample of caffeine, c8h10n4o2, burns in a constant-volume calorimeter that has a heat capaci...
Questions





Mathematics, 23.08.2019 13:10



Physics, 23.08.2019 13:10

History, 23.08.2019 13:10



English, 23.08.2019 13:10

Biology, 23.08.2019 13:10


Geography, 23.08.2019 13:10


World Languages, 23.08.2019 13:10


Mathematics, 23.08.2019 13:10

Mathematics, 23.08.2019 13:10