
Chemistry, 09.11.2019 00:31 haydencheramie
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you have in front of you 100 ml of 7.00×10−2 m hcl, 100 ml of 5.00×10−2 m naoh, and plenty of distilled water. you start to add hcl to a beaker of water when someone asks you a question. when you return to your dilution, you accidentally grab the wrong cylinder and add some naoh. once you realize your error, you assess the situation. you have 84.0 ml of hcl and 88.0 ml of naoh left in their original containers. part a assuming the final solution will be diluted to 1.00 l , how much more hcl should you add to achieve the desired ph?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you h...
Questions

English, 13.07.2020 22:01



Mathematics, 13.07.2020 22:01

Biology, 13.07.2020 22:01










Physics, 13.07.2020 22:01




Biology, 13.07.2020 22:01

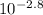 = [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³
= [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³

