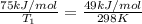Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the following reaction equation. 2h202(aq) →2h20(/) + 02(g) the activation energy for this reaction is 75 kj/mol. in the presence of a metal catalyst the activation energy is lowered to 49 kj/mol. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the metal-catalyzed reaction at 25°c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3



Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the following...
Questions


English, 07.04.2021 17:20

English, 07.04.2021 17:20

English, 07.04.2021 17:20

Mathematics, 07.04.2021 17:20



Mathematics, 07.04.2021 17:20



Chemistry, 07.04.2021 17:20

Mathematics, 07.04.2021 17:20






Biology, 07.04.2021 17:20

Chemistry, 07.04.2021 17:20

Mathematics, 07.04.2021 17:20




 ..........(1)
..........(1) = activation energy for non-catalyzed reaction = 75 kJ/mol
= activation energy for non-catalyzed reaction = 75 kJ/mol = activation energy for catalyzed reaction = 49 kJ/mol
= activation energy for catalyzed reaction = 49 kJ/mol = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ? = temperature for catalyzed reaction =
= temperature for catalyzed reaction =