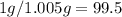Chemistry, 09.11.2019 01:31 journeyburks07
Questions: (1 pt each) remember these questions will be checked for completion only. if you have questions about them, make sure to ask your instructor. 1. suppose the material that you are recrystallizing fails to crystallize out of the cold solvent. what would you do to recover the material from solution? 2. suppose you obtain 1.0g of product from the hydrolysis of 1.3 ml of methyl salicylate. calculate your % yield. 3. describe the characteristics of a good recrystallization solvent. explain how the rate of crystal growth can affect the purity of a recrystallized

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Questions: (1 pt each) remember these questions will be checked for completion only. if you have qu...
Questions


Mathematics, 10.12.2020 01:10

History, 10.12.2020 01:10

History, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10

Social Studies, 10.12.2020 01:10



Chemistry, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10





Mathematics, 10.12.2020 01:10

Mathematics, 10.12.2020 01:10


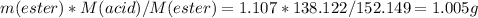 so the yield will be
so the yield will be