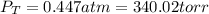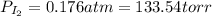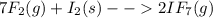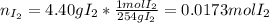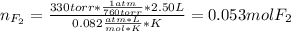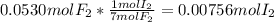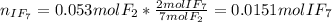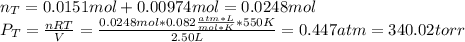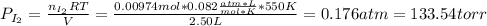Chemistry, 09.11.2019 05:31 jimennacastillo15
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine heptafluoride forms.3.30 × 102 torr of f2 and 4.40 g of solid i2 are put into a 2.50 l container at 2.50 × 102 k and the container is heated to 5.50 × 10^2 k.
(a) what is the final pressure?
ptotal =
(b) what is the partial pressure of i2 gas?
pi2 = i2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine hep...
Questions

Mathematics, 17.03.2021 23:40


English, 17.03.2021 23:40






Computers and Technology, 17.03.2021 23:40

Social Studies, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

English, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

Health, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Social Studies, 17.03.2021 23:40

Health, 17.03.2021 23:40