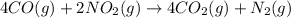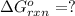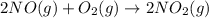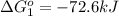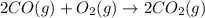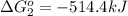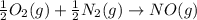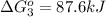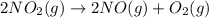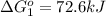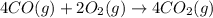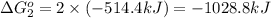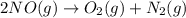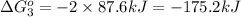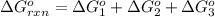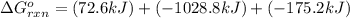Chemistry, 10.11.2019 01:31 TheOriginal2x
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reactions and given δg∘rxn values: a) 2no(g)+o2(g)→2no2(g), δg∘rxn= - 72.6 kjb) 2co(g)+o2(g)→2co2(g), δg∘rxn= - 514.4 kjc) 12o2(g)+12n2(g)→no(g), δg∘rxn= 87.6 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2
You know the right answer?
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reaction...
Questions

Mathematics, 26.01.2021 04:00






Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00


Mathematics, 26.01.2021 04:00





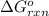 for the reaction is -1131.4 kJ
for the reaction is -1131.4 kJ