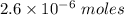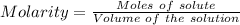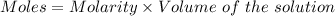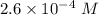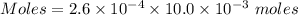Chemistry, 10.11.2019 03:31 shaylakabler333
Watch the video that shows using the dilution method to make a solution of known concentration. note that while the video shows the use of a beaker, in the lab we typically use a volumetric flask to make the diluted solution. the stock solution of kmno4 has a concentration of 2.6 × 10-4 m. the pipette has a volume of 10.0 ml. what is the amount of kmno4 delivered to the solution, in moles

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

You know the right answer?
Watch the video that shows using the dilution method to make a solution of known concentration. note...
Questions

Mathematics, 22.02.2021 22:40

Arts, 22.02.2021 22:40




Social Studies, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40







Mathematics, 22.02.2021 22:40


English, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40


Mathematics, 22.02.2021 22:40