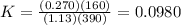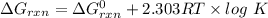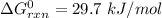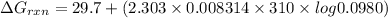Chemistry, 10.11.2019 05:31 elijahjwhite15
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentrations, calculate the free energy change for this reaction at 37.0 °c (310 k). δg°\' for the reaction is 29.7 kj/mol. assume that the reaction occurs at ph 7. [malate] = 1.13 mm [oxaloacetate] = 0.270 mm [nad ] = 390 mm [nadh] = 160 mm

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

You know the right answer?
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentra...
Questions

Mathematics, 05.02.2021 18:30

World Languages, 05.02.2021 18:30

Mathematics, 05.02.2021 18:30

English, 05.02.2021 18:30




Mathematics, 05.02.2021 18:30

Mathematics, 05.02.2021 18:30





Chemistry, 05.02.2021 18:30





Mathematics, 05.02.2021 18:30

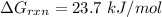
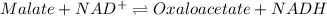
![K =\frac {[oxaloacetate][NADH]}{[malate][NAD^+]}](/tpl/images/0367/7081/e135a.png)
![[NAD^+]](/tpl/images/0367/7081/f5e9f.png) = 390 mM
= 390 mM