
Chemistry, 10.11.2019 05:31 battlemarshmell
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . if of sodium sulfate is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of sodium sulfate. be sure your answer has the correct number of significant digits in it.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions

English, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00



Social Studies, 21.09.2021 01:00



Mathematics, 21.09.2021 01:00

English, 21.09.2021 01:00


World Languages, 21.09.2021 01:00

History, 21.09.2021 01:00



Biology, 21.09.2021 01:00



English, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00

Advanced Placement (AP), 21.09.2021 01:00


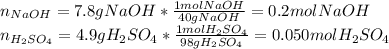
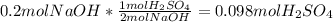
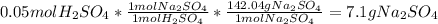
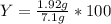
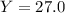 %
%

