
Chemistry, 10.11.2019 05:31 jalenclarke25
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonae]/[carbonic acid] = 0.11.b) [bicarbonate]/[carbonic acid] = 9.4.c) [bicarbonate]/[carbonic acid] = 0.38.d) none of the above ratios is correct. e) [bicarbonate]/[carbonic acid] = 2.65.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions







Mathematics, 22.06.2019 01:10








Mathematics, 22.06.2019 01:10





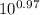 = [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4
= [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4

