Chemistry, 10.11.2019 06:31 Alienchild239
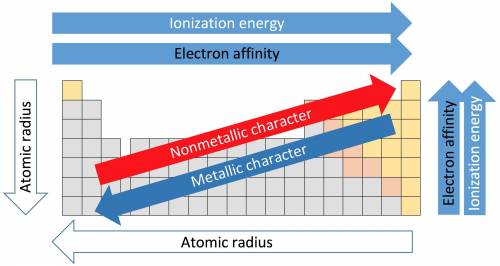
How do the periodic trends in metallic character compare to those for ionization energy? how do the periodic trends in metallic character compare to those for ionization energy? metals tend to have higher ionization energies than nonmetals. metals tend to have lower ionization energies than nonmetals. metals and nonmetals tend to have the same ionization energies.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
How do the periodic trends in metallic character compare to those for ionization energy? how do the...
Questions


History, 07.11.2019 18:31


English, 07.11.2019 18:31


Mathematics, 07.11.2019 18:31


History, 07.11.2019 18:31

Social Studies, 07.11.2019 18:31


History, 07.11.2019 18:31

Chemistry, 07.11.2019 18:31



Mathematics, 07.11.2019 18:31



History, 07.11.2019 18:31


Mathematics, 07.11.2019 18:31