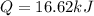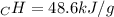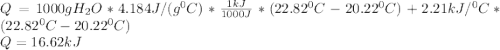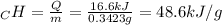Chemistry, 10.11.2019 06:31 angelica9613
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorimeter and the 1.000kg of water contained therein rose from 20.22 degrees celcius to 22.82 degrees celcius. the heat capacity of the calorimeter is 2.21 kj/c. the heat capacity of water = 4.184 j/g c. a. how much heat was given off during combustion fo the sample of pentane. answer = 16.6 kjb. what is the heat of combustion, in kilojoules, per gram of pentaneanswer = 48.6 kj/g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorim...
Questions






Mathematics, 03.12.2019 23:31


Mathematics, 03.12.2019 23:31

Biology, 03.12.2019 23:31




Biology, 03.12.2019 23:31

Mathematics, 03.12.2019 23:31

Chemistry, 03.12.2019 23:31

Mathematics, 03.12.2019 23:31