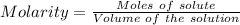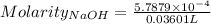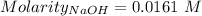Chemistry, 10.11.2019 06:31 maddietomlinson113
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of potassium hydrogen phthalate (khc8h4o4, often abbreviated khp), an acid with one acidic hydrogen and a molar mass of 204.220 g/mol. it takes 36.01 ml of a sodium hydroxide solution to titrate a 0.1182-g sample of khp. what is the molarity of the sodium hydroxide

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of pot...
Questions

English, 17.03.2020 00:25


History, 17.03.2020 00:25


Mathematics, 17.03.2020 00:26

History, 17.03.2020 00:26


Mathematics, 17.03.2020 00:26

Mathematics, 17.03.2020 00:26

French, 17.03.2020 00:26



Mathematics, 17.03.2020 00:26






English, 17.03.2020 00:26

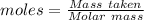
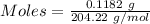
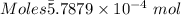
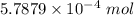 of KHP reacts with
of KHP reacts with