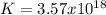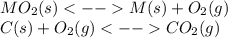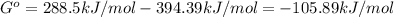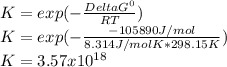Chemistry, 10.11.2019 07:31 lovelyheart5337
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g) delta g = 288.5 kj/mol
1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s)
2.) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions




Mathematics, 07.12.2019 09:31



Mathematics, 07.12.2019 09:31


Mathematics, 07.12.2019 09:31


Mathematics, 07.12.2019 09:31



Mathematics, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

Chemistry, 07.12.2019 09:31



English, 07.12.2019 09:31