Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
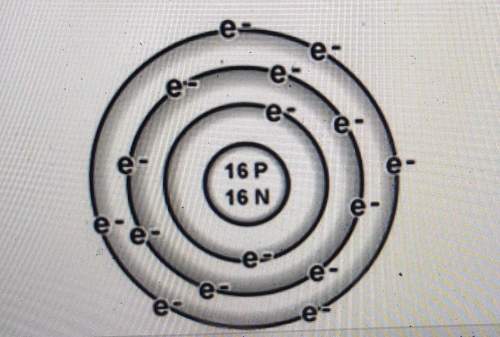
Is the atom below more likely to gain electrons or to lose electrons? explain how you can tell.
Questions


Mathematics, 28.09.2019 07:00

Mathematics, 28.09.2019 07:00








Business, 28.09.2019 07:00

History, 28.09.2019 07:00




Mathematics, 28.09.2019 07:00

Social Studies, 28.09.2019 07:00


Health, 28.09.2019 07:00

Mathematics, 28.09.2019 07:00