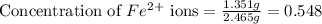Chemistry, 11.11.2019 20:31 ashleyremon
The fe2 (55.845 g/mol) content of a 2.465 g steel sample dissolved in 50.00 ml was determined by tiration with a standardized 0.140 m potassium permanganate (kmno4, 158.034 g/mol) solution. the titration required 34.59 ml to reach the end point. what is the concentration of iron in the steel sample? express your answer as grams of fe per grams of steel (g fe2 / g steel).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

You know the right answer?
The fe2 (55.845 g/mol) content of a 2.465 g steel sample dissolved in 50.00 ml was determined by tir...
Questions

Mathematics, 04.03.2021 14:00

Health, 04.03.2021 14:00


Advanced Placement (AP), 04.03.2021 14:00

English, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

English, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00


Social Studies, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

Social Studies, 04.03.2021 14:00



Mathematics, 04.03.2021 14:00




English, 04.03.2021 14:00

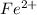 ions is
ions is 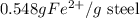
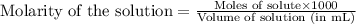
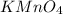 = 0.140 M
= 0.140 M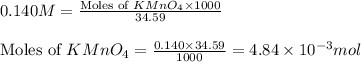
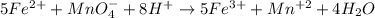
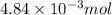 moles of permanganate ions will react with =
moles of permanganate ions will react with = 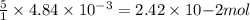 of iron (II) ions.
of iron (II) ions.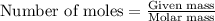
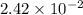 moles
moles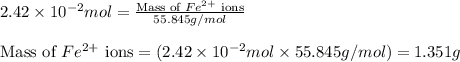
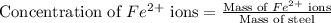
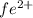 ions = 1.351 g
ions = 1.351 g