Chemistry, 11.11.2019 21:31 pedroramirezr2
The "air bags" that are currently installed in automobiles to prevent injuries in the event of a crash are equipped with sodium azide, nan₃, which decomposes when activated by an electronic igniter to produce nitrogen gas that fills the bag. how many liters of nitrogen, measured at 25°c and 1.00 atm, will be produced by 100.0 g of nan₃?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
The "air bags" that are currently installed in automobiles to prevent injuries in the event of a cra...
Questions

History, 06.10.2019 06:30

Mathematics, 06.10.2019 06:30


Mathematics, 06.10.2019 06:30

Mathematics, 06.10.2019 06:30

English, 06.10.2019 06:30

Biology, 06.10.2019 06:30



Mathematics, 06.10.2019 06:30



History, 06.10.2019 06:30



Mathematics, 06.10.2019 06:30


Physics, 06.10.2019 06:30

History, 06.10.2019 06:30

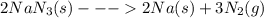
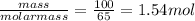
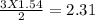 mol
mol