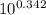Phosphoric acid is a triprotic acid with the following pka values: pka1=2.148, pka2=7.198, pka3=12.375you wish to prepare 1.000 l of a 0.0500 m phosphate buffer at ph 7.540. to do this, you choose to mix the two salt forms involved in the second ionization, nah2po4 and na2hpo4, in a 1.000 l volumetric flask and add water to the mark. what mass of each salt will you add to the mixture? mass nah2po4mass na2hpo4what other combination of phosphoric acid and/or its salts could be mixed to prepare this buffer? (check all that apply).h3po4 and nah2po4h3po4 and na2hpo4h3po4 and na3hpo4nah2po4 and na3po4na2hpo4 and solve and explain

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2

Chemistry, 23.06.2019 14:00
Ineed in question 5and6 in science the subject is science
Answers: 1
You know the right answer?
Phosphoric acid is a triprotic acid with the following pka values: pka1=2.148, pka2=7.198, pka3=12.3...
Questions


History, 04.10.2019 22:00




Mathematics, 04.10.2019 22:00



Geography, 04.10.2019 22:00


Mathematics, 04.10.2019 22:00

History, 04.10.2019 22:00

Biology, 04.10.2019 22:00


Mathematics, 04.10.2019 22:00