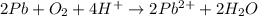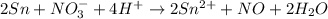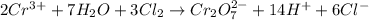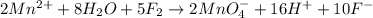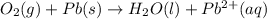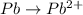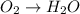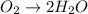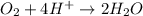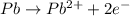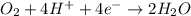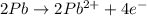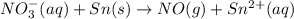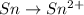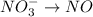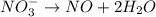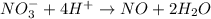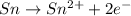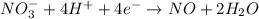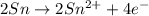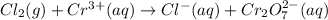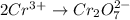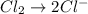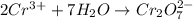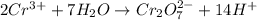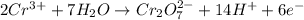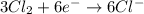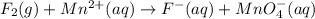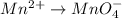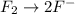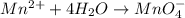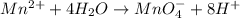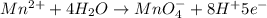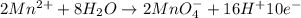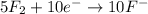Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-reaction method.
(use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
o2(g) + pb(s) → h2o(l) + pb2+(aq) (b) no3−(aq) + sn(s) → no(g) + sn2+(aq) (c) cl2(g) + cr3+(aq) → cl −(aq) + cr2o72−(aq) (d) f2(g) + mn2+(aq) → f −(aq) + mno4−(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-...
Questions

Advanced Placement (AP), 04.03.2021 05:30

Mathematics, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30

English, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30


Mathematics, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30


Mathematics, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30

Mathematics, 04.03.2021 05:30






English, 04.03.2021 05:30