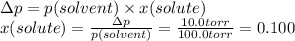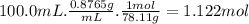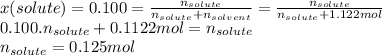Chemistry, 11.11.2019 23:31 robert7248
The solubility of gas in a liquid is profoundly affected by pressure. this relation is expressed by henry's law, which states that the solubility of a gas in a liquid, expressed in moles per liter (or m), at a given temperature is directly proportional to the partial pressure of the gas over the solution. this relation can be expressed mathematically as solubility=k⋅p. the constant k is characteristic of the specific gas and p is the partial pressure of the gas over the solution usually expressed in atmospheres. the constant k usually has units of moles per liter per atmosphere and is reported at 25 ∘c. this expression can be used to calculate any of the three variables provided that the other two are known for any gas.1) air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25.0 ∘c, then2 component will dissolve in water with a solubility of 4.88×10−4 m. what is the value of henry's law constant for n2 under these conditions? 2) the vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 ∘c. assuming raoult’s law is obeyed, how many moles of a nonvolatile solute must be added to 100.0 ml of benzene to decrease its vapor pressure by 10.0% at 26.1 ∘c? the density of benzene is 0.8765 g/cm3.3) a 2.650×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of naclin water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.3 ml . the density of water at 20.0∘c is 0.9982 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
The solubility of gas in a liquid is profoundly affected by pressure. this relation is expressed by...
Questions








History, 30.08.2019 05:00

English, 30.08.2019 05:00


English, 30.08.2019 05:00

Mathematics, 30.08.2019 05:00



Computers and Technology, 30.08.2019 05:00

Mathematics, 30.08.2019 05:00

Health, 30.08.2019 05:00

Mathematics, 30.08.2019 05:00

Social Studies, 30.08.2019 05:00