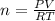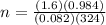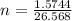Chemistry, 12.11.2019 04:31 ChloeLiz7111
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required to form 1.6 l of o2 at a temperature of 324 k and a pressure of 0.984 atm ?
express your answer using two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2


Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
Questions


Mathematics, 06.10.2019 22:50

Social Studies, 06.10.2019 22:50

Computers and Technology, 06.10.2019 22:50



Spanish, 06.10.2019 23:00



Biology, 06.10.2019 23:00



Mathematics, 06.10.2019 23:00


Social Studies, 06.10.2019 23:00


Business, 06.10.2019 23:00


Biology, 06.10.2019 23:00