
Chemistry, 12.11.2019 04:31 CameronVand21
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is generally reported as ppm caco3 . to measure water hardness, a sample of groundwater is titrated with edta, a chelating agent, in the presence of the indicator eriochrome black t, symbolized as in. eriochrome black t, a weaker chelating agent than edta, is red in the presence of ca2+ and turns blue when ca2+ is removed. red blue ca(in)2+ + edta ⟶ ca(edta)2+ + in a 50.00 ml sample of groundwater is titrated with 0.0450 m edta . if 13.70 ml of edta is required to titrate the 50.00 ml sample, what is the hardness of the groundwater in molarity and in parts per million of caco3 by mass? assume that ca2+ accounts for all of the hardness in the groundwater.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is...
Questions

Mathematics, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10


Mathematics, 16.04.2021 02:10



Mathematics, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10

Spanish, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10

Arts, 16.04.2021 02:10


Mathematics, 16.04.2021 02:10

Computers and Technology, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10


Mathematics, 16.04.2021 02:10

Mathematics, 16.04.2021 02:10

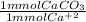 * 100.09 mg/mmol = 61.70 mg CaCO₃
* 100.09 mg/mmol = 61.70 mg CaCO₃

