
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of analysis to determine its exact concentration of fe2+ ions. as part of the analysis a 15 ml sample of this solution was diluted to a final volume of 100 ml. the iron (ii) ion concentration of the diluted solution was found to be 1.35 x 10-3 mg/ml fe2+. what was the total number of milligrams of iron (ii) that were contained in the original solution?
note that this question is not asking for how many mg/ml of iron (ii) where in the original solution, but the total mass of iron contained in the original solution. hint: you must first calculate the mg/ml of iron (ii) in the original solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1
You know the right answer?
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of ana...
Questions




Social Studies, 15.12.2019 12:31


Social Studies, 15.12.2019 12:31


English, 15.12.2019 12:31


Biology, 15.12.2019 12:31

Mathematics, 15.12.2019 12:31


Mathematics, 15.12.2019 12:31



History, 15.12.2019 12:31




Mathematics, 15.12.2019 12:31

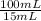 = 9,00x10⁻³ mg/mL
= 9,00x10⁻³ mg/mL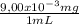 = 2,25 mg of Fe²⁺
= 2,25 mg of Fe²⁺

