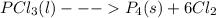Chemistry, 12.11.2019 06:31 gabrielolivas59
4pcl₃(l)  p₄(s) + 6cl₂(g); δh= 304.0 kj
p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown, which statement is true?
a) when 1 mol pcl₃(l) reacts, 304.0 kj are released. b) when 1 mol p₄(s) is produced, 304.0 kj are consumed. c) when 548.56 g pcl₃(l) react, 304.0 kj are released. d) when 137.14 g pcl₃(l) react, 304.0 kj are consumed. e) when 123.88 g p₄(s) are produced, 304.0 kj are released.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
4pcl₃(l) [tex]\longrightarrow[/tex] p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown...
based on the reaction shown...
Questions

English, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01













English, 03.08.2020 14:01

Social Studies, 03.08.2020 14:01