Chemistry, 12.11.2019 21:31 jeremyrs101
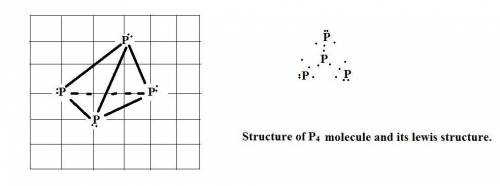
The standard state of phosphorus at 25∘c is p4. this molecule has four equivalent p atoms, no double or triple bonds, and no expanded octets. draw its lewis structure. draw the molecule by placing atoms on the grid and connecting them with bonds. include all non-bonding electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2

Chemistry, 23.06.2019 17:30
What amount in moles does 242 l of carbon dioxide occupy at 1.32 atm and 20 degrees c?
Answers: 2
You know the right answer?
The standard state of phosphorus at 25∘c is p4. this molecule has four equivalent p atoms, no double...
Questions


History, 01.10.2019 04:30

Mathematics, 01.10.2019 04:30

Mathematics, 01.10.2019 04:30

History, 01.10.2019 04:30

Biology, 01.10.2019 04:30



History, 01.10.2019 04:30

History, 01.10.2019 04:30

Mathematics, 01.10.2019 04:30

Computers and Technology, 01.10.2019 04:30


Mathematics, 01.10.2019 04:30

Biology, 01.10.2019 04:30


History, 01.10.2019 04:30


Health, 01.10.2019 04:30

World Languages, 01.10.2019 04:30

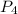 molecule.the
molecule.the 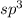 hybridized phosphorus atom lies in the corner of the regular tetrahedron. Each phosphorus atom is connected to three other phosphorus atoms.
hybridized phosphorus atom lies in the corner of the regular tetrahedron. Each phosphorus atom is connected to three other phosphorus atoms.