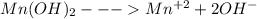Chemistry, 12.11.2019 23:31 chassidytjtrimb
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a solution buffered to be ph = 6.5 a solution buffered to be ph = 3.5 pure water > ph 3.5 > ph 6.5 (least soluble) ph 3.5 > ph 6.5 > pure water (least soluble) ph 3.5 > pure water > ph 6.5 (least soluble) pure water > ph 6.5 > ph 3.5 (least soluble) ph 6.5 > ph 3.5 > pure water (least soluble)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

You know the right answer?
Compare the solubility of mn(oh)2 (ksp = 1.6x10-13)in the following solutions: pure water, a soluti...
Questions

Mathematics, 02.05.2021 02:10

English, 02.05.2021 02:10



Computers and Technology, 02.05.2021 02:10




History, 02.05.2021 02:10

English, 02.05.2021 02:10








Mathematics, 02.05.2021 02:10