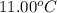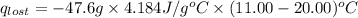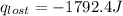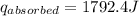A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water was 20.00°c. after the compound dissolved, the temperature of the water was 11.00°c. assume the heat was completely absorbed from the water and no heat was absorbed by the reaction container or the surroundings. calculate the heat absorbed by the process. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water w...
Questions



Business, 19.09.2019 20:30

Mathematics, 19.09.2019 20:30

Mathematics, 19.09.2019 20:30

Mathematics, 19.09.2019 20:30

Mathematics, 19.09.2019 20:30

Mathematics, 19.09.2019 20:30


Chemistry, 19.09.2019 20:30


Social Studies, 19.09.2019 20:30

History, 19.09.2019 20:30

Geography, 19.09.2019 20:30


English, 19.09.2019 20:30


Social Studies, 19.09.2019 20:30


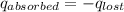
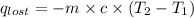
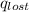 = heat lost by the water = ?
= heat lost by the water = ?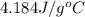
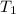 = initial temperature of water =
= initial temperature of water = 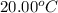
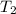 = final temperature of water =
= final temperature of water =