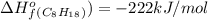Chemistry, 13.11.2019 00:31 ridzrana02
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat per mole of c8h18(g)c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5099.5 kj/mol c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh rxn°=−5099.5 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat...
Questions

History, 18.07.2019 22:00



Social Studies, 18.07.2019 22:00



History, 18.07.2019 22:00



Mathematics, 18.07.2019 22:00




Biology, 18.07.2019 22:00


Mathematics, 18.07.2019 22:00

Biology, 18.07.2019 22:00



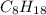 is -222 kJ/mol
is -222 kJ/mol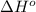
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0371/3800/45485.png)
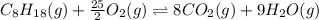
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_8H_{18})}\times \Delta H^o_f_{(C_8H_{18})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0371/3800/70c63.png)
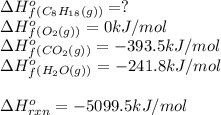
![-5099.5=[(8\times -393.5)+(9\times -241.5)]-[(1\times \Delta H^o_f_{(C_8H_{18})}))+(\frac{25}{2}\times 0)]](/tpl/images/0371/3800/1a9fc.png)