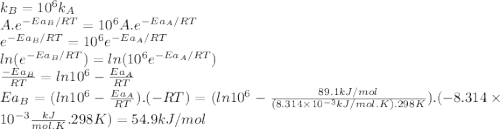Chemistry, 13.11.2019 02:31 joseperez1224
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k. reaction b is one million times faster than reaction a at the same temperature. the products of each reaction are 10.0 kj mol–1 (2.39 kcal mol–1) more stable than the reactants. (a) what is the standard free energy of activation of reaction b?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

You know the right answer?
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k....
Questions

Mathematics, 12.12.2021 04:00


Mathematics, 12.12.2021 04:00

Mathematics, 12.12.2021 04:00




Mathematics, 12.12.2021 04:00



Mathematics, 12.12.2021 04:00

Mathematics, 12.12.2021 04:00


Mathematics, 12.12.2021 04:00


English, 12.12.2021 04:00

Mathematics, 12.12.2021 04:00

Mathematics, 12.12.2021 04:00


Biology, 12.12.2021 04:00

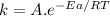
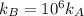 .
.