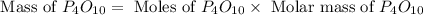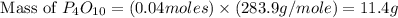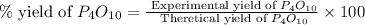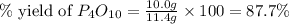Chemistry, 13.11.2019 02:31 gonzalezant8428
The phophorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by nurning phosphorus in oxygen.
(a)what is the limiting reactant when 0.200 mol of p4 and and 0.200 mol of o2 react according to p4 + 5o2 ? p4o10
(b)calculate the percent yield if 10.0 g of p4o10 is isolated from the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
The phophorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by nurning...
Questions

English, 12.04.2021 16:50


Mathematics, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50

Spanish, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50






Advanced Placement (AP), 12.04.2021 16:50

English, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50

Mathematics, 12.04.2021 16:50

English, 12.04.2021 16:50


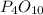 is, 87.7 %
is, 87.7 %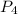 = 0.200 mole
= 0.200 mole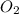 = 0.200 mole
= 0.200 mole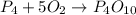
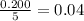 moles of
moles of