
Choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. 0.075 m kno2 0.075 m licn 0.075 m (nh4)3po4 0.075 m nai 0.075 m nabro4

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Choose the aqueous solution below with the lowest freezing point. these are all solutions of nonvola...
Questions

Biology, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01



Mathematics, 26.08.2020 01:01


Biology, 26.08.2020 01:01



Computers and Technology, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01

Social Studies, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01



Mathematics, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01

English, 26.08.2020 01:01

English, 26.08.2020 01:01





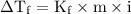
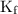 ) so that what affects the value of
) so that what affects the value of  is the value of i
is the value of i


