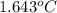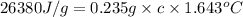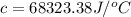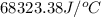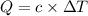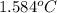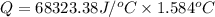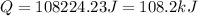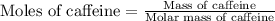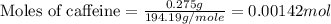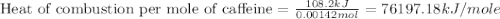Chemistry, 13.11.2019 04:31 tamaraquirozmorales
When a 0.235-g sample of benzoic acid is combusted in a bomb calorimeter, the temperature rises 1.643 ∘c . when a 0.275-g sample of caffeine, c8h10o2n4, is burned, the temperature rises 1.584 ∘c . using the value 26.38 kj/g for the heat of combustion of benzoic acid, calculate the heat of combustion per mole of caffeine at constant volume.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
When a 0.235-g sample of benzoic acid is combusted in a bomb calorimeter, the temperature rises 1.64...
Questions

History, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00


Mathematics, 19.04.2021 19:00

English, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00


Mathematics, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00


Arts, 19.04.2021 19:00


Mathematics, 19.04.2021 19:00



Physics, 19.04.2021 19:00


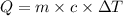
 = change in temperature =
= change in temperature =