
Chemistry, 13.11.2019 04:31 friendsalwaysbae
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g) hexane is a low-boiling point component of gasoline. answer the question in part 1 through part 3, if the rate of reaction of c6h14 is 1.95 mol l-1s-1. the rate of reaction of c6h14 is the rate of disappearance of c6h14. what was the rate of formation of co2?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2


Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g)...
Questions


Computers and Technology, 22.07.2019 15:50




Mathematics, 22.07.2019 15:50

History, 22.07.2019 15:50

English, 22.07.2019 15:50


Social Studies, 22.07.2019 15:50

Biology, 22.07.2019 15:50


Biology, 22.07.2019 15:50

Spanish, 22.07.2019 15:50

Mathematics, 22.07.2019 15:50


Mathematics, 22.07.2019 15:50

Mathematics, 22.07.2019 15:50


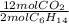 = 11,7 M/s
= 11,7 M/s

