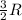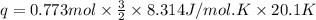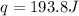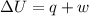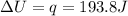A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough heat is applied to raise the temperature of the gas by 20.1 k. assuming ideal gas behavior, calculate the amount of heat in joules (q) required to affect this temperature change and the total change in internal energy, ? u. note that some books use ? e as the symbol for internal energy instead of ? u.
q= ? u=

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
A0.773 mol sample of xe(g) initially at 298 k and 1.00 atm is held at constant volume while enough h...
Questions


Business, 25.01.2021 02:40

Social Studies, 25.01.2021 02:50

Mathematics, 25.01.2021 02:50

Chemistry, 25.01.2021 02:50


Mathematics, 25.01.2021 02:50



World Languages, 25.01.2021 02:50

Mathematics, 25.01.2021 02:50

Mathematics, 25.01.2021 02:50

Biology, 25.01.2021 02:50

Mathematics, 25.01.2021 02:50



History, 25.01.2021 02:50


Biology, 25.01.2021 02:50

Biology, 25.01.2021 02:50

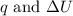 is 193.8 J and 193.8 J respectively.
is 193.8 J and 193.8 J respectively.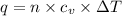
 = Change in temperature = 20.1 K
= Change in temperature = 20.1 K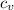 = heat capacity at constant volume of Xe (mono-atomic molecule) =
= heat capacity at constant volume of Xe (mono-atomic molecule) =