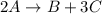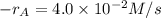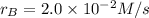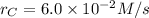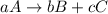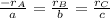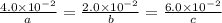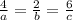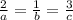Chemistry, 13.11.2019 05:31 ninilizovtskt
At a certain time in a reaction, substance a is disappearing at a rate of 4.0×10−2 m/s, substance b is appearing at a rate of 2.0×10−2 m/s, and substance c is appearing at a rate of 6.0×10−2 m/s. which of the following could be the stoichiometry for the reaction being studied?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
At a certain time in a reaction, substance a is disappearing at a rate of 4.0×10−2 m/s, substance b...
Questions



















Mathematics, 17.04.2020 15:47