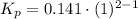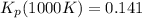Chemistry, 13.11.2019 06:31 SisterMina
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar, h2(g) is introduced into the reaction vessel. the total pressure is held constant at 1 bar and at equilibrium the composition of the mixture in mole percent is h2 : 26% ; c2h4: 26% ; c2h6 : 48%calclate kp at 1000 k.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar...
Questions


Mathematics, 16.04.2021 22:00



Mathematics, 16.04.2021 22:00

English, 16.04.2021 22:00



History, 16.04.2021 22:00


Mathematics, 16.04.2021 22:00



Mathematics, 16.04.2021 22:00

English, 16.04.2021 22:00


Mathematics, 16.04.2021 22:00


Social Studies, 16.04.2021 22:00

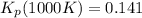
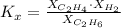
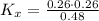
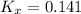
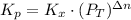
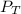 : total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant
: total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant