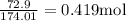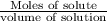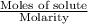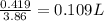Chemistry, 13.11.2019 06:31 nuconteaza119
How many liters of a 3.86 m k2so4 solution are needed to provide 72.9 g of k2so4 (molar mass 174.01 g/mol)? recall that m is equivalent to mol/l.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
How many liters of a 3.86 m k2so4 solution are needed to provide 72.9 g of k2so4 (molar mass 174.01...
Questions



Mathematics, 17.12.2019 12:31

Social Studies, 17.12.2019 12:31


Mathematics, 17.12.2019 12:31

History, 17.12.2019 12:31

Physics, 17.12.2019 12:31




Social Studies, 17.12.2019 12:31


Mathematics, 17.12.2019 12:31


Biology, 17.12.2019 12:31

Mathematics, 17.12.2019 12:31

Mathematics, 17.12.2019 12:31

Mathematics, 17.12.2019 12:31

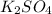 will be needed
will be needed