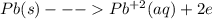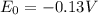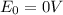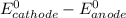Chemistry, 13.11.2019 18:31 Gghbhgy4809
When lead metal is added to a beaker of hcl(aq), a gas is produced. knowing that lead is oxidized and that hydrogen is reduced, write the balanced net equation for the reaction. (use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.) how many electrons are transferred in the balanced equation? what quantity of useful work can be obtained when pb is added directly to the beaker of hcl?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 12:30
When utilizing a transmission electron microscope, why is it necessary to stain the specimen with heavy metal salts?
Answers: 1
You know the right answer?
When lead metal is added to a beaker of hcl(aq), a gas is produced. knowing that lead is oxidized an...
Questions




Mathematics, 02.02.2022 14:00

Geography, 02.02.2022 14:00

Social Studies, 02.02.2022 14:00


Mathematics, 02.02.2022 14:00





Mathematics, 02.02.2022 14:00

Mathematics, 02.02.2022 14:00

Mathematics, 02.02.2022 14:00

Mathematics, 02.02.2022 14:00



Mathematics, 02.02.2022 14:00