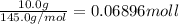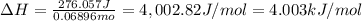Chemistry, 13.11.2019 19:31 danbat3023
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs when a solid dissolves in water. a 10.0 g sample of an ionic compound with a molar mass of 145.0 g/mol was added to a sample of deionized water to produce 60.0 grams of solution. after stirring and dissolving the solid, the temperature was found to change from 25.00 ∘c to 23.89 ∘c . calculate the enthalpy of solution, δhsoln , per mole of salt dissolved. assume the specific heat of the solution is 4.06 j/(g⋅∘c ) and the heat capacity of the calorimeter is 5.10 j/ ∘c . calculate the heat change experienced by the calorimeter contents, . = j calculate the heat change experienced by the calorimeter, . = j calculate the heat change produced by the solution process, . = j calculate δhsoln , the enthalpy of solution for one mole of solid in kilojoules per mole. δhsoln= kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

You know the right answer?
Acoffee cup calorimeter was used to measure the heat of solution, the change in enthalpy that occurs...
Questions

Biology, 17.10.2021 01:30



Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

English, 17.10.2021 01:30

History, 17.10.2021 01:30


Chemistry, 17.10.2021 01:30

History, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30

English, 17.10.2021 01:30

History, 17.10.2021 01:30

World Languages, 17.10.2021 01:30

Mathematics, 17.10.2021 01:30


History, 17.10.2021 01:30

Arts, 17.10.2021 01:30

Chemistry, 17.10.2021 01:30

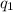
 = change in temperature = -1.11°C
= change in temperature = -1.11°C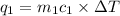
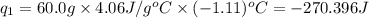
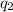
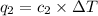
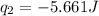
![Q=[-q_1+(-q_2)]](/tpl/images/0372/7156/e1e62.png)
![Q=[-(m_1c_1\times \Delta T)+(-c_2\times \Delta T)]](/tpl/images/0372/7156/ff7dd.png)
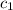 = specific heat of solution =
= specific heat of solution = 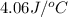
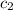 = specific heat of calorimeter=
= specific heat of calorimeter= 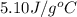
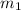 = mass of solution= 60.0 g
= mass of solution= 60.0 g![Q=[-(60.0 g\times 4.06J/g^oC\times (-1.11)^oC)+(- 5.10J/^oC\times (-1.11)^oC)]](/tpl/images/0372/7156/5fbbc.png)
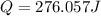
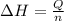
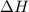 = enthalpy change = ?
= enthalpy change = ?