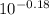Chemistry, 14.11.2019 03:31 THEOUTCOMER
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl must be added to this buffer solution to change the ph by 0.18 units? assume the total volume remains unchanged at 400 ml. for h2po4-, ka2 = 6.3 × 10^-8.
o 0.063 mol
o 1.0 mol
o 0.025 mol
o 0.082 mol
o 0.50 mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl...
Questions

English, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20






English, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20



English, 06.11.2020 22:20

Business, 06.11.2020 22:20

Biology, 06.11.2020 22:20

History, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20