
Chemistry, 14.11.2019 04:31 jeanine239
Astudent who is performing this experiment pours an 8.50 ml sample of the saturated borax solution into a 10 ml graduated cylinder after the borax solution had cooled to a certain temperature t. the student rinses the sample into a small beaker using distilled water, and then titrates the solution with a 0.500 m hcl solution. 12.00 ml of the hcl solution is needed to reach the endpoint of the titration.
calculate the value of ksp for borax at temperature t.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
12.00 moles of naclo3 will produce how many grams of o2? a) 256 g of o2 b) 576 g of o2 c) 288 g of o2
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Astudent who is performing this experiment pours an 8.50 ml sample of the saturated borax solution i...
Questions

Biology, 25.11.2020 21:00

Social Studies, 25.11.2020 21:00

Biology, 25.11.2020 21:00

Computers and Technology, 25.11.2020 21:00

Arts, 25.11.2020 21:00

Mathematics, 25.11.2020 21:00

Social Studies, 25.11.2020 21:00

Mathematics, 25.11.2020 21:00

English, 25.11.2020 21:00


Health, 25.11.2020 21:00

Mathematics, 25.11.2020 21:00





Physics, 25.11.2020 21:00

Health, 25.11.2020 21:00

Mathematics, 25.11.2020 21:00

Arts, 25.11.2020 21:00

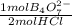 = 3,00x10⁻³ mol of B₄O₇²⁻
= 3,00x10⁻³ mol of B₄O₇²⁻

