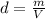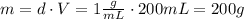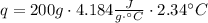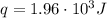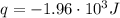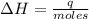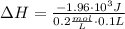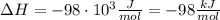Chemistry, 14.11.2019 06:31 lexybellx3
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueous ammonia in a constant-pressure calorimeter of negligible heat capacity. the following reaction occurs when the two solutionsare mixedhcl(aq)+ nh3(> nh4cl(aq)the temperature increase is 2.34°c. calculate heat change of the reaction per mole of hcl reacted. assume that the densities and specific heats of the solutions are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueou...
Questions

Biology, 30.08.2019 13:10


History, 30.08.2019 13:10

Chemistry, 30.08.2019 13:10

English, 30.08.2019 13:10

English, 30.08.2019 13:10

Mathematics, 30.08.2019 13:10

English, 30.08.2019 13:10

Mathematics, 30.08.2019 13:10

English, 30.08.2019 13:10

Mathematics, 30.08.2019 13:10

History, 30.08.2019 13:10



Mathematics, 30.08.2019 13:10



Mathematics, 30.08.2019 13:10


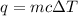 (1)
(1)