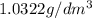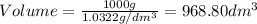1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929 g dm–3, and 1.0322 g dm–3, respectively. a) assume a sample of mass 1000 g, and calculate the volume at each temperature. b) from these data, and assuming that air obeys charles’s law, determine a value for th

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929...
Questions

Mathematics, 28.08.2020 23:01








Biology, 28.08.2020 23:01



Mathematics, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01



Biology, 28.08.2020 23:01




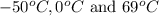 are
are 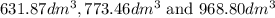 respectively.
respectively.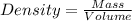
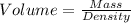
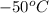 :
: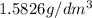
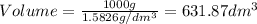
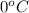 :
: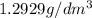
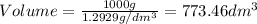
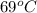 :
: