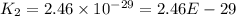Chemistry, 14.11.2019 06:31 strawberrymochi390
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be the rate constant at 119 k if the activation energy is 80. kj/mol? this is a second order reaction, giving k the units of m-1s-1 this will not change with the change in temperature. do not include units in your answer. exponential numbers need to be entered like this: 2 e-1 means 2 x 10-1. the rate constant, k, at 119 k equals:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3
You know the right answer?
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be t...
Questions

Mathematics, 02.07.2020 02:01



Mathematics, 02.07.2020 02:01




Mathematics, 02.07.2020 02:01






Physics, 02.07.2020 02:01





Chemistry, 02.07.2020 02:01

Mathematics, 02.07.2020 02:01

![Rate=1.4\times 10^{-2}[NO_2]^2](/tpl/images/0373/7605/5818c.png) ..........(1)
..........(1)![Rate=k[NO_2]^2](/tpl/images/0373/7605/d48ef.png) ............(2)
............(2)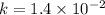
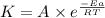
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0373/7605/6d953.png)
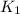 = rate constant at
= rate constant at 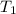 =
= 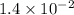
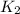 = rate constant at
= rate constant at 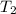 = ?
= ?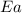 = activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole
= activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole![\log (\frac{K_2}{1.4\times 10^{-2}})=\frac{80000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{500}-\frac{1}{119}]](/tpl/images/0373/7605/44fe7.png)