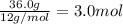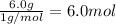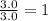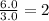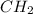Chemistry, 14.11.2019 17:31 KindaSmartPersonn
A42.0g sample of a compound containing only c and h was analyzed. the results showed that the sample contained 36.0g of c and 6.0g of h. which of the following questions about the compound can be answered using the results of the analysis?
a) what was the volume of the sample?
b) what is the molar mass of the compound?
c) what is the chemical stability of the compound?
d) what is the empirical formula of the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
A42.0g sample of a compound containing only c and h was analyzed. the results showed that the sample...
Questions

Mathematics, 14.01.2021 22:20



Physics, 14.01.2021 22:20

Health, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20


Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Chemistry, 14.01.2021 22:20



Mathematics, 14.01.2021 22:20


Mathematics, 14.01.2021 22:20