Pls
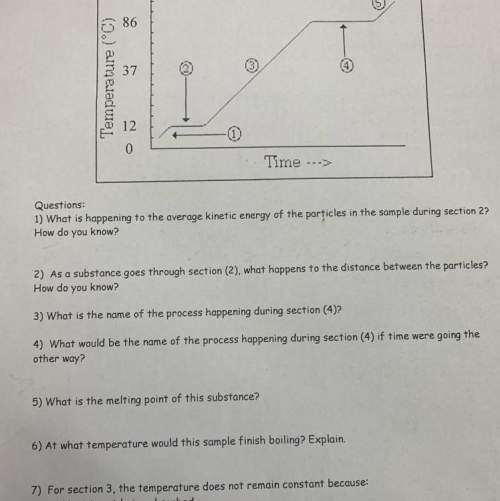
questions:
1) what is happening to the average kinetic energy of the particles in...

Chemistry, 14.11.2019 18:31 bellabae8390
Pls
questions:
1) what is happening to the average kinetic energy of the particles in the sample during section 2?
how do you know?
martno da
2) as a substance goes through section (2), what happens to the distance between the particles?
how do you know?
2 a
3
3) what is the name of the process happening during section (4)?
4) what would be the name of the process happening during section (4) if time were going the
other way?
5) what is the melting point of this substance?
6) at what temperature would this sample finish boiling? explain.
7) for section 3, the temperature does not remain constant because:
a. heat is not being absorbed
b. the ice is colder that the water
c. heat energy is being converted to potential energy
d. heat energy is being converted to kinetic energy

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Questions

Computers and Technology, 02.03.2020 19:16








Chemistry, 02.03.2020 19:17




Mathematics, 02.03.2020 19:17


Computers and Technology, 02.03.2020 19:17


English, 02.03.2020 19:17


Social Studies, 02.03.2020 19:17



