
Chemistry, 23.08.2019 20:40 hbkakabryce0p3fkoq
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions




Mathematics, 24.05.2021 23:30

Mathematics, 24.05.2021 23:30

Mathematics, 24.05.2021 23:30

Mathematics, 24.05.2021 23:30

Physics, 24.05.2021 23:30


Mathematics, 24.05.2021 23:30

Social Studies, 24.05.2021 23:30


Mathematics, 24.05.2021 23:30


Chemistry, 24.05.2021 23:30





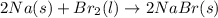
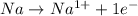
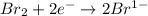
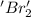 is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is, 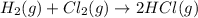
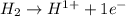

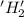 is oxidized and
is oxidized and 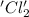 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 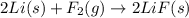
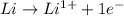
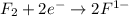
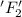 is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is, 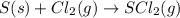
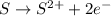
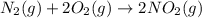
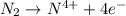

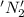 is oxidized and
is oxidized and 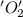 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 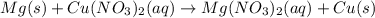
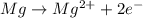
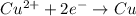
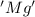 is oxidized and
is oxidized and 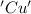 is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.
is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.

