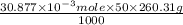Chemistry, 14.11.2019 20:31 SucMaDongShan
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (free acid) required to prepare 50 ml of a 100 mm buffer that is ph = 7.20. the pka of hepes is 7.55 at 20° c. the formula weight of the sodium salt is 260.31. the formula weight of the free acid is 238.31. weigh out the appropriate amounts of the hepes (sodium salt) and hepes (free acid), transfer to a 100 ml beaker, dissolve in deionized water to an approximate volume of 40 ml

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (fre...
Questions

History, 12.09.2019 20:10



Biology, 12.09.2019 20:10


History, 12.09.2019 20:10

Chemistry, 12.09.2019 20:10


Geography, 12.09.2019 20:10



Mathematics, 12.09.2019 20:10

Mathematics, 12.09.2019 20:10



Chemistry, 12.09.2019 20:10

English, 12.09.2019 20:10

History, 12.09.2019 20:10


![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0374/5521/81f72.png)
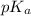 = 7.55.
= 7.55.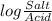
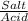 = 0.4467
= 0.4467 = 100 mM
= 100 mM 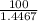
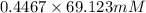
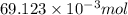 .
.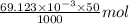
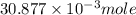 .
.