Chemistry, 14.11.2019 20:31 unknown54321
The value of δ°′δg°′ for the conversion of glucose-6-phosphate to fructose-6-phosphate (f6p) is +1.67 kj/mol+1.67 kj/mol . if the concentration of glucose-6-phosphate at equilibrium is 1.85 mm1.85 mm , what is the concentration of fructose-6-phosphate? assume a temperature of 25.0°c25.0°c .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
The value of δ°′δg°′ for the conversion of glucose-6-phosphate to fructose-6-phosphate (f6p) is +1.6...
Questions

Mathematics, 17.04.2021 08:30




Business, 17.04.2021 08:30

History, 17.04.2021 08:30

Mathematics, 17.04.2021 08:30


English, 17.04.2021 08:40

Mathematics, 17.04.2021 08:40

Computers and Technology, 17.04.2021 08:40


Mathematics, 17.04.2021 08:40

Mathematics, 17.04.2021 08:40


History, 17.04.2021 08:40



Mathematics, 17.04.2021 08:40

Mathematics, 17.04.2021 08:40

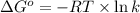
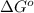 = standard Gibbs free energy = +1.67 kJ/mol = +1670 J/mol
= standard Gibbs free energy = +1.67 kJ/mol = +1670 J/mol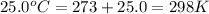
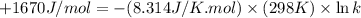
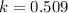
![K=\frac{[\text{fructose-6-phosphate}]}{[\text{glucose-6-phosphate}]}](/tpl/images/0374/4842/feaba.png)
![0.509=\frac{[\text{fructose-6-phosphate}]}{1.85mM}](/tpl/images/0374/4842/d0c84.png)