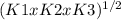Chemistry, 14.11.2019 22:31 HaJEReMY5170
Determine the value of the equilibrium constant, kgoal, for the reaction n2(g)+h2o(g)⇌no(g)+12n2h4(g), kgoal=? by making use of the following information: 1. n2(g)+o2(g)⇌2no(g), k1 = 4.10×10−31 2. n2(g)+2h2(g)⇌n2h4(g), k2 = 7.40×10−26 3. 2h2o(g)⇌2h2(g)+o2(g), k3 = 1.06×10−10express your answer numerically.
kgoal

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reaction n2(g)+h2o(g)⇌no(g)+12n2h4(g...
Questions


History, 28.10.2020 22:40


Mathematics, 28.10.2020 22:40


History, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40



Physics, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40


Mathematics, 28.10.2020 22:40


English, 28.10.2020 22:40


Mathematics, 28.10.2020 22:40


Biology, 28.10.2020 22:40